Abstract
Background: Covalent (c) Bruton tyrosine kinase inhibitors (BTKi) have markedly improved outcomes in several B-cell malignancies. Despite this progress, treatment discontinuation due to cBTKi intolerance remains a significant barrier, ultimately limiting efficacy. Pirtobrutinib, a highly selective, non-covalent (reversible) BTKi, inhibits both wildtype and C481-mutant BTK with equal low nM potency, and has favorable oral pharmacology that enables continuous BTK inhibition throughout the dosing interval regardless of intrinsic rate of BTK turnover. Pirtobrutinib is well tolerated and has demonstrated promising efficacy in patients (pts) with poor-prognosis B-cell malignancies following prior therapy, including prior cBTKi (Mato et al. Lancet, 2021). Here, we evaluated the safety and tolerability of pirtobrutinib monotherapy specifically in pts with relapsed/refractory (R/R) B-cell malignancies who were intolerant to a prior cBTKi.
Methods: For this analysis, pts with R/R B-cell malignancies enrolled to the multicenter phase 1/2 BRUIN study (NCT03740529) with documented intolerance to prior cBTKi were analyzed. Intolerance was defined as having discontinued any prior cBTKi due to persistent/recurrent adverse events as assessed by the physician, in the absence of progressive disease. Pirtobrutinib monotherapy was administered once daily in 28-day cycles until disease progression or discontinuation due to toxicity. Analyses were performed descriptively.
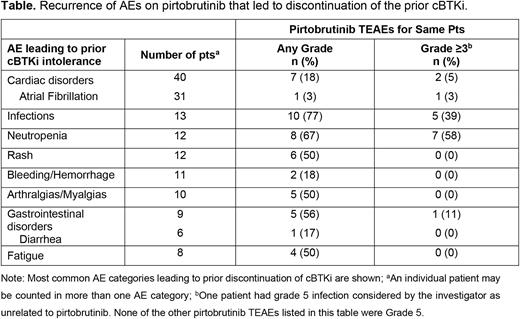
Results: As of 31 January 2022, 566 pts (CLL/SLL, n=276; MCL, n=150; other NHL, n=140) treated with pirtobrutinib had received a prior cBTKi-containing regimen (prior lines of cBTKi containing regimens: 1 regimen, n=454; 2 regimens, n=93; ≥3 regimens, n=19). Among these 566 pts, 123 (22%) pts (CLL/SLL, n=79; MCL, n=20; other NHL, n=24) discontinued a prior cBTKi due to toxicity. This cBTKi intolerant cohort had received one or more of the following: ibrutinib (n=118, 96%), acalabrutinib (n=29, 24%), or zanubrutinib (n=6, 5%). At the time of enrollment to BRUIN, the median age for pts with prior cBTKi intolerance was 70 years (IQR: 64-75), and ECOG PS was 0, 1 and 2 in 58%, 35%, and 7% of pts, respectively. The most common adverse events (AEs) leading to prior cBTKi discontinuation were cardiac disorders [n=40, 33%; primarily atrial fibrillation (n=31, 25%)], infections (n=13, 11%), neutropenia (n=12, 10%), rash (n=12, 10%), bleeding/hemorrhage (n=11, 9%), arthralgias/myalgias (n=10, 8%), gastrointestinal disorders [n=9, 7%; diarrhea (n=6, 5%)], and fatigue (n=8, 7%). In these 123 pts with prior cBTKi intolerance, median time on pirtobrutinib treatment was 11 months (IQR, 4-18), with 63% (n=78) of pts remaining on pirtobrutinib at the time of data cut-off date. Of the 45 pts who had discontinued pirtobrutinib, the majority did so for progressive disease (62%, n=28). Overall, 9 (7%) pts discontinued pirtobrutinib for AEs which showed no particular pattern. Discontinuations related to pirtobrutinib occurred in 4 pts (1 each) including: myalgia, neutropenia, rash maculo-papular, and staphylococcal sepsis, and discontinuations unrelated to pirtobrutinib occurred in 5 pts (1 each) including: anxiety, abdominal pain and blood alkaline phosphatase increase, chronic respiratory failure, pneumonia, and COVID-19 and dyspnea. Treatment-emergent adverse events (TEAEs) on pirtobrutinib were manageable with dose reductions in 10 pts (8%) primarily due to neutropenia (n=6). The median pirtobrutinib relative dose intensity was 98% (IQR, 92-100). Pirtobrutinib TEAEs recurring in the same patient and same AE category as those leading to prior cBTKi intolerance are listed in the Table. Overall recurrence of a grade ≥3 pirtobrutinib TEAE in the same patient and category as that leading to prior cBTKi discontinuation was observed in only 13% (n=16/123) of pts.
Conclusions: Pirtobrutinib monotherapy was safe and well-tolerated in the majority of pts with B-cell malignancies with documented intolerance to prior cBTKi therapy. Most pts did not experience high-grade recurrence of the AE that led to discontinuation of the prior cBTKi, and among those who did, none discontinued pirtobrutinib for this AE. Pirtobrutinib may be an important consideration to extend the benefit of BTK inhibition among pts with intolerance to a prior cBTKi.
Disclosures
Shah:Miltenyi Biotec: Consultancy, Research Funding; Novartis: Consultancy; Kite Pharma: Consultancy; TG therapeutics: Consultancy; Incyte Corporation: Consultancy, Honoraria, Speakers Bureau; Lilly Oncology: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy; Epizyme: Consultancy. Wang:MJH Life Sciences: Honoraria; InnoCare: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Loxo Oncology: Research Funding; Eastern Virginia Medical School: Honoraria; Dava Oncology: Honoraria; Moffit Cancer Center: Honoraria; OncLive: Honoraria; LLC TS Oncology: Honoraria; Studio ER Congressi: Honoraria; Medscape: Honoraria; Practice Point Communications (PPC): Honoraria; Genmab: Research Funding; Physicians Education Resources (PER): Honoraria; VelosBio: Consultancy, Research Funding; Celgene: Research Funding; Vinverx: Research Funding; Juno Therapeutics: Consultancy, Research Funding; Meeting Minds Experts: Honoraria; IDEOlogy Health: Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Oncology Specialty Group: Honoraria; Molecular Templates: Research Funding; Oncternal: Consultancy, Research Funding; Pepromene Bio: Consultancy; Milken Institute: Consultancy; Merck: Honoraria; Lilly: Consultancy, Research Funding; Leukemia & Lymphoma Society: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Deciphera: Consultancy; BioInvent: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Acerta Pharma: Honoraria, Research Funding; AbbVie: Consultancy. Brown:BeiGene, Gilead, Loxo/Lilly, MEI Pharma, SecuraBio, Sun, TG Therapeutics: Research Funding; Abbvie, Acerta/Astra-Zeneca, BeiGene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Eli Lilly, Genentech/Roche, Hutchmed, iOnctura, Janssen, MEI Pharma, Pharmacyclics: Consultancy. Patel:TG Therapeutics: Consultancy, Speakers Bureau; Velos Bio: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; CRISPR Therapeutics: Research Funding; Epizyme: Consultancy, Research Funding; Fate Therapeutics: Research Funding; Caribou Biosciences: Consultancy; Celgene: Consultancy, Research Funding, Speakers Bureau; Sunesis Pharmaceuticals: Research Funding; Pharmacyclics/Janssen: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; BeiGene: Consultancy; Nurix: Research Funding; MEI Pharma: Consultancy, Research Funding; Morphosys: Consultancy; Kite pharma: Consultancy, Research Funding, Speakers Bureau; Genetech/Roche: Consultancy, Research Funding, Speakers Bureau; Trillium Therapuetics/Pfizer: Consultancy, Research Funding; Xencor: Consultancy, Research Funding; Abbvie: Consultancy; Adaptive Biotechnologies: Research Funding; Curis, Inc: Research Funding; Loxo Oncology: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Aptevo Therapeutics: Research Funding. Woyach:MorphoSys: Consultancy, Research Funding; Genentech: Consultancy; Schrodinger: Research Funding; Pharmacyclics: Consultancy; Newave: Consultancy; BeiGene: Consultancy; Loxo@Lilly: Research Funding; ArQule: Consultancy; AbbVie: Consultancy, Research Funding; Karyopharm Therapeutics: Research Funding; Janssen: Consultancy; AstraZeneca: Consultancy. Wierda:Genzyme: Consultancy; Bristol Meyers Squibb (Juno and Celgene): Research Funding; Kite, a Gilead Company: Research Funding; Xencor: Research Funding; Miragen: Research Funding; Sanofi: Consultancy; Juno: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Sunesis: Research Funding; Cyclacel: Research Funding; Genentech: Research Funding; AstraZeneca/Acerta Pharma. Inc.: Research Funding; Pharmacyclics LLC: Research Funding; Gilead Sciences: Research Funding; AbbVie: Research Funding; Karyopharm: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Janssen: Research Funding; GSK/Novartis: Research Funding. Ujjani:Loxo Oncology: Consultancy, Research Funding; Astara: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly and Company: Consultancy, Research Funding; Genentech: Consultancy; Beigene: Consultancy; Abbvie: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Adaptive Biotechnologies: Research Funding; Incyte: Consultancy. Eyre:Loxo Oncology @ Lilly: Membership on an entity's Board of Directors or advisory committees, Other, Speakers Bureau; PeerView: Speakers Bureau; Medscape: Speakers Bureau; Kite Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Zinzani:Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; University of Bologna: Current Employment. Alencar:TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Loxo Oncology: Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; OncLive: Membership on an entity's Board of Directors or advisory committees; SeaGen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees. Gastinne:Gilead/Kite, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings/travel, participation in a data safety monitoring board or advisory board. Ghia:Janssen: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Lilly/Loxo: Consultancy, Honoraria; BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding. Lamanna:Loxo Oncology/Eli Lilly and Company: Research Funding; Genentech: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mingsight: Research Funding; AstraZenenca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Octapharma: Research Funding; Oncternal: Research Funding; TG Therapeutics: Research Funding. Hoffmann:Pharmacyclics: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria. Patel:Prelude Therapeutics: Research Funding; Ribon Therapeutics: Research Funding; Seven and Eight Biopharmaceuticals: Research Funding; Syndax: Research Funding; Taiho Pharmaceutical: Research Funding, Speakers Bureau; Takeda: Research Funding; Tesaro: Research Funding; TopAlliance BioSciences Inc: Research Funding; Vigeo: Research Funding; ORIC: Research Funding; Artios: Research Funding; Treadwell: Research Funding; MabSpace: Research Funding; IgM Biosciences: Research Funding; Puretech: Research Funding; BioTheryX: Research Funding; Black Diamond Therapeutics: Research Funding; NGM Biopharmaceuticals: Research Funding; Novartis: Research Funding; Nurix: Research Funding; Relay Therapeutics: Research Funding; Samumed: Research Funding; Silicon Therapeutics: Research Funding; TeneoBio: Research Funding; Zymeworks: Research Funding; Olema: Research Funding; Adagene: Research Funding; Astellas: Research Funding; Accutar Biotech: Research Funding; Compugen: Research Funding; Immunogen: Research Funding; Blueprint Pharmaceuticals: Research Funding; Pharmacyclics: Honoraria; Bayer: Honoraria; Adaptive Biotechnologies: Honoraria; Exelixis: Speakers Bureau; ION Pharma: Other: Leadership; Portola Pharmaceuticals: Research Funding; Placon: Research Funding; Pfizer: Honoraria, Research Funding; Moderna Therapeutics: Research Funding; Mirati Therapeutics: Research Funding; Millennium: Research Funding; Merck: Research Funding; Macrogenics: Research Funding; Lycera: Research Funding; LSK Biopartners: Research Funding; Loxo: Research Funding; Kymab: Research Funding; Klus Pharma: Research Funding; Janssen: Honoraria, Research Funding; Jacobio: Research Funding; Incyte: Research Funding; Ignyta: Research Funding; Hutchison MediPharma: Research Funding; Hengrui Therapeutics: Research Funding; H3 Biomedicine: Research Funding; GlaxoSmithKline: Research Funding; Gilead Sciences: Research Funding; Genentech/Roche: Honoraria, Research Funding, Speakers Bureau; FORMA Therapeutics: Research Funding; Evelo Therapeutics: Research Funding; EMD Serono: Research Funding; Eli Lilly and Company: Research Funding; Daiichi Sankyo: Research Funding; Cyteir Therapeutics: Research Funding; Clovis Oncology: Research Funding; CicloMed: Research Funding; Checkpoint Therapeutics: Research Funding; Celgene: Research Funding, Speakers Bureau; Boehringer Ingelheim: Research Funding; BioNTech AG: Research Funding; AstraZeneca: Research Funding; Aileron Therapeutics: Research Funding; Agenus: Research Funding; ADC Therapeutics: Research Funding; Acerta Pharma: Research Funding; Pfizer/EMD Serono: Consultancy; Pharmacyclics/Janssen: Consultancy. Flinn:Kite Pharma: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Servier Pharmaceuticals: Consultancy; Genmab: Consultancy; AstraZeneca: Consultancy, Research Funding; InnoCare Pharma: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Celgene: Research Funding; Trillium Therapeutics: Research Funding; IGM Biosciences: Research Funding; Incyte: Research Funding; Novartis: Consultancy, Research Funding; Hutchison MediPharma: Consultancy; MorphoSys: Consultancy, Research Funding; Pfizer: Research Funding; Iksuda Therapeutics: Consultancy; Portola Pharmaceuticals: Research Funding; Rhizen Pharmaceuticals: Research Funding; Janssen: Consultancy, Research Funding; Nurix Therapeutics: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Curis: Research Funding; Constellation Pharmaceuticals: Research Funding; Loxo@Lilly: Research Funding; Infinity Pharmaceuticals: Research Funding; Myeloid Therapeutics: Research Funding; Merck: Research Funding; Gilead Sciences: Research Funding; Secura Bio: Consultancy; Agios: Research Funding; ArQule: Research Funding; Vincerx Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Research Funding; Verastem: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Xencor: Consultancy; Biopath: Research Funding; Bristol Myers Squibb: Research Funding; CALIBR: Research Funding; CALGB: Research Funding; BeiGene: Consultancy, Research Funding; Century Therapeutics: Consultancy; Forty Seven: Research Funding; Forma Therapeutics: Research Funding; City of Hope National Medical Center: Research Funding; Seattle Genetics: Research Funding; Unum Therapeutics: Research Funding; TG Therapeutics: Consultancy, Research Funding; Takeda: Consultancy; CTI Biopharma: Research Funding; Fate Therapeutics: Research Funding; Epizyme: Research Funding; Millenium Pharmaceuticals: Research Funding; Tessa Therapeutics: Research Funding; TCR2 Therapeutics: Research Funding; Triphase Research & Development Corp: Research Funding; 2seventy bio: Research Funding. Gerson:Loxo Oncology: Research Funding; Abbvie: Consultancy; Genentech: Consultancy. Ma:Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Research Funding; Juno: Research Funding; Janssen: Consultancy, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy; BeiGene: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding; Loxo: Research Funding. Coombs:MEI Pharma: Honoraria; AbbVie: Consultancy, Honoraria, Speakers Bureau; Beigene: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; CTI Biopharma: Current equity holder in publicly-traded company; Novartis: Honoraria; TG Therapeutics: Honoraria; Loxo/Lilly: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria. Cheah:Gilead: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Eli Lilly and Company: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Merck Sharp & Dohme: Consultancy, Honoraria. Fakhri:TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Consultancy; University of California San Francisco: Current Employment; Abbvie: Membership on an entity's Board of Directors or advisory committees; Adaptive: Research Funding; Angiocrine: Research Funding; BMC: Research Funding; Astrazeneca: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Lox/o Oncology/Eli Lilly and Company: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees. Kim:Takeda: Honoraria; Sanofi: Research Funding; Beigene: Research Funding; Kyowa-kirin: Research Funding; Boryong: Research Funding; Donga: Research Funding. Barve:Tempus Time Program: Membership on an entity's Board of Directors or advisory committees; Tempus: Consultancy; Texas Oncology: Current Employment. Cohen:BMS/Celgene: Research Funding; BeiGene: Consultancy, Research Funding; Janssen: Consultancy; Genentech: Research Funding; Novartis: Research Funding; Kite Pharma/Gilead: Consultancy; HutchMed: Consultancy, Research Funding; Astrazeneca: Consultancy, Research Funding; Aptitude Health: Consultancy; Lilly Oncology/Eli Lilly: Consultancy, Research Funding; Takeda: Research Funding. Jurczak:Mei Pharma: Consultancy; Janssen: Consultancy, Research Funding; Eli Lilly and Company: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Celgene: Research Funding; TgTherapeutica: Research Funding; AstraZeneca: Consultancy; Maria Sklodowska-Curie National Research Institue of Oncology: Current Employment. Munir:Janssen, AstraZeneca, Alexion, Sobi, Novartis, Roche, Abbvie, Gilead: Honoraria; Janssen, AstraZeneca, Alexion, Abbvie, Novartis, Roche: Membership on an entity's Board of Directors or advisory committees. Thompson:VJHemOnc: Honoraria; Intellisphere, LLC: Honoraria; Brazilian Association of Hematology and Hemotherapy: Honoraria; MJH Life Sciences: Honoraria; Massachusetts Medical Society: Honoraria; Curio Science: Honoraria. Roeker:Janssen: Consultancy; Loxo Oncology: Consultancy, Other: Travel support, Research Funding; Pharmacyclics: Consultancy; Pfizer: Consultancy, Research Funding; TG Therapeutics: Consultancy; Abbott Laboratories: Current equity holder in publicly-traded company; Aptose Biosciences: Research Funding; Ascentage: Consultancy; Qilu Puget Sound Biotherapeutics: Research Funding; Beigene: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months. Bao:Loxo@Lilly: Current Employment, Current equity holder in publicly-traded company. Cangemi:Loxo@Lilly: Current Employment; Eli Lilly and Company: Current equity holder in publicly-traded company. Kherani:Loxo@Lilly: Current Employment, Current equity holder in publicly-traded company. Walgren:Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Han:Loxo@Lilly: Current Employment. Ruppert:Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Mato:Nurix: Research Funding; Genentech: Honoraria, Research Funding; Dava: Honoraria; Pharmacyclics, LLC: Honoraria, Research Funding; Octopharma: Honoraria, Research Funding; Acerta: Research Funding; AbbVie: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Curio: Honoraria; Adaptive Biotechnologies: Honoraria; Pfizer: Research Funding; Janssen: Honoraria, Research Funding; DTRM Biopharma: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; TG Therapeutics, Inc: Honoraria, Research Funding; Genmab: Honoraria, Research Funding; Johnson & Johnson: Honoraria, Research Funding; LOXO: Honoraria, Research Funding; BMS: Honoraria; Medscape: Honoraria; PER: Honoraria; PerView: Honoraria.
OffLabel Disclosure:
We will be presenting data from the BRUIN trial. Pirtobrutinib is not approved yet.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal